Abstract
Introduction:Chronic kidney disease (CKD) is common in sickle cell disease (SCD). We have recently reported on the progression of CKD in SCD and factors associated with it (Ciccone EJ et al, ASH, 2016). The purpose of this study was to evaluate the prevalence of rapid decline in kidney function, factors associated with such decline, and the association of rapid decline in kidney function with mortality in adult patients with SCD.
Methods: We conducted a retrospective study of patients seen between July 2004 and December 2013 at an adult Sickle Cell Clinic. Patients had confirmed diagnoses of SCD, were at least 18 years old, and were in non-crisis state. We excluded patients with histories of HIV, hepatitis B and C, or systemic lupus erythematosus. Clinical and laboratory variables were obtained from medical records. Estimated glomerular filtration rate (eGFR) was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation. Rapid decline of kidney function was defined as eGFR loss of >3.0 ml/min/1.73 m2per year during the observation period.
Logistic regression was used to model the association of rapid eGFR decline with clinical and laboratory variables, adjusting for age and sex. Multivariable analysis with variable selection was performed with backward elimination from the following initial variables - hemoglobin, reticulocyte count, lactate dehydrogenase, baseline eGFR, history of stroke, hydroxyurea therapy, systolic blood pressure, use of ACE inhibitors/angiotensin receptor blockers (ACE-I/ARB) and history of diabetes. Since proteinuria measures were missing for many patients, we performed sensitivity analyses with 1) exclusion of proteinuria, 2) inclusion of only patients with available values, 3) assignment of no proteinuria to those missing values, or 4) assignment of proteinuria to those missing values. Age- and sex- adjusted Cox proportional hazards models were used to evaluate the association of the slope of eGFR (continuous variable) or rapid decline in eGFR (binary variable) with mortality. The slope of eGFR was estimated by linear regression modeling of eGFR over time. Kaplan-Meier estimates of survival probabilities for rapid and non-rapid decline groups were obtained, and the log-rank test was used to compare the survival probabilities for the two groups.
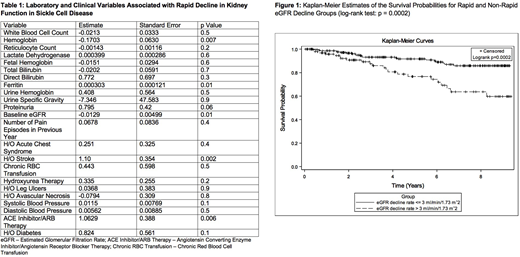
Results: Three hundred and thirty-one SCD patients with at least two eGFR measurements(SS = 218, SC = 67, Sβ0= 18, Sβ+= 22, SE = 2, SD = 2, SHPFH = 2), median age of 29 years (IQR: 20 - 41 years) were evaluated and followed for a median of 4.01 years (IQR: 1.66, 7.19). Rapid decline of eGFR (>3.0 ml/min/1.73 m2per year) was noted in 103 (31.1%) patients; 80 (33.9%) with severe genotype (HbSS/HbSβ0thalassemia) and 21 (23.6%) with mild genotype (HbSC/HbSβ+thalassemia).Baseline laboratory factors significantly associated with rapid decline in eGFR were hemoglobin (p = 0.007), ferritin (p = 0.01), and baseline eGFR (p = 0.01) (Table 1). There was a trend towards an association (p = 0.06) with baseline proteinuria (at least 1+ on dipstick urinalysis). Clinical variables significantly associated with eGFR decline were history of stroke (p = 0.002) and use of ACE-I/ARB (p = 0.006). In multivariable analysis, history of stroke (estimate: 1.07, p = 0.01) and use of ACE-I/ARB (estimate: 1.15, p = 0.01) were associated with rapid eGFR decline when proteinuria was excluded from the model or proteinuria status was assigned to those with missing values. When we limited the dataset to those with available values for proteinuria, only history of stroke (estimate: 2.00, p = 0.007) was associated with rapid decline in eGFR. Finally, we observed an association of the slope of eGFR change over time with mortality (hazard ratio [HR]: 0.99, p = 0.0002). Rapid decline in eGFR was significantly associated with increased mortality compared with eGFR decline of ≤ 3 ml/min/1.73 m2per year (HR: 2.40, p = 0.005). Kaplan-Meier estimates also showed significantly lower survival probabilities for patients with rapid eGFR decline (log-rank test; p = 0.0002) (Figure 1).
Conclusion:Rapid decline in eGFR is common in SCD. Use of ACE-I/ARB and history of stroke are associated with rapid decline in renal function. Rapid decline in eGFR is associated with an increased risk of death in SCD. Long-term studies are required to determine if therapies that may reduce loss of kidney function may decrease mortality in SCD.
Ataga:Pfizer: Research Funding; Bioverativ: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals: Honoraria; Modus Therapeutics: Honoraria; Global Blood Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal